Editor’s note
On 1/22/15, a web address was updated on this page and in the PDF.
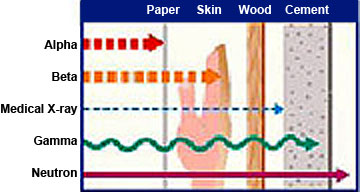 The penetrating power of radiation. (Image courtesy of the U.S. Nuclear Regulatory Commission)
The penetrating power of radiation. (Image courtesy of the U.S. Nuclear Regulatory Commission)
Types of radiation
There are four types of radiation released from atoms: alpha, beta, gamma and neutron radiation.
Alpha particles are highly charged and the heaviest of the nuclear radiations. Because of their size and weight they are unable to travel very far and have a limited ability penetrate. They cannot travel more than four to seven inches in the air and can be stopped by a sheet of paper or skin. They can be a hazard if they are inhaled or swallowed.
Beta particles are smaller and travel faster than alpha particles. They can travel several feet in the air and are able to penetrate skin, though they do not usually penetrate deep enough to reach vital organs. They can be stopped by a thin sheet of metal or plastic or a block of wood.
Gamma rays are not particles, but waves of radioactive energy. They travel much further and have more penetrating power than either alpha or beta particles. They can travel as much as a mile in open air and it takes several feet of concrete or several inches of a dense material such as lead to block them.
Neutron radiation occurs when nuclear particles collide with other materials. Neutrons have an exceptional ability to penetrate other materials and are extremely hazardous. Fortunately, this type of radiation is generally only found in a nuclear power plant where it is shielded by steel, concrete and several feet of water.
Radiation can enter the body in the following ways:
- Inhalation
Gaseous or airborne particles, dust particulates, and matter with radioactive material may enter the body through the lungs. Remember that air itself is not radioactive; radiation is contained in particles carried by the air. - Ingestion
Internal radioactive contamination may enter the body through the gastrointestinal tract by way of contaminated food or drink and by swallowing contaminated mucous from the nasal area. - Absorption
Radioactive material may be absorbed through the skin or mucous membranes. - Puncture or injection
Radioactive material can penetrate the body through cuts, wounds, and punctures in the skin.
Reducing radiation exposure
Time, distance and shielding are the three primary methods of reducing or eliminating exposure to radioactive materials.
- Time
Minimize time spent near a radioactive source or radioactive contamination. The less time exposed to the source of radiation, the lower the dose received. - Distance
Maximize the distance from a radioactive source or radioactive contamination. Keep as much distance as possible between oneself and the source of radiation. The farther one is from the source, the lower the dose received. - Shielding
Shielding simply means having something that will absorb radiation between you and the source of the radiation. Keep as much protection between oneself and the source as possible.
What should I do if there is a radioactive incident near me?
The first and most important rule is: Listen to and follow the instructions of your local emergency personnel. Emergency personnel have been trained in how to respond in the event of an incident, including those involving radiological materials. They will provide instructions on how to keep yourself and your family safe.
Shouldn’t I just try to get as far away from the radiation source as possible?
Not necessarily. In a radiological incident, quite often residents will be instructed to remain in their homes, a concept known as “shelter-in-place.” The reason for this is that, if an incident involves alpha or beta particles, your home will provide a tremendous amount of safety as it will block the penetration of these particles. Move to an interior room with few windows or the basement. Turn off all air conditioners and ventilation systems. If you have the materials available, you should seal any cracks in your home where particles may be able to enter. Duct tape and plastic sheeting work well for this purpose. Although you will have to open up the room occasionally to allow fresh air in, you will likely receive much less exposure than if you left your home where you might inhale radioactive materials. According to the Federal Emergency Management Agency (FEMA), “Ten square feet of floor space per person will provide sufficient air to prevent carbon dioxide build-up for up to five hours, assuming a normal breathing rate while resting.”
How can I prepare for an emergency or disaster?
For all disasters, you and your family can take the following three steps, which will be extremely helpful.
- Put together an emergency kit.
Your emergency kit should contain enough materials to sustain you, your family, and those who may shelter with you for a minimum of three days. In addition to food, water and other supplies, you should include a battery-powered or hand-crank radio and a NOAA weather radio with tone alert, and extra batteries for both. At a minimum, you should check your emergency kit every six months.- MU Extension publication EMW1012, Disaster supplies kit
- List of recommended items to include in an emergency kit, Ready (Federal Emergency Management Agency) (URL updated 1/22/15)
- Develop and practice a family emergency plan.
Know where in your home to go during an emergency and how to contact members of your family. For all emergencies you should have a plan for if you stay at home or if you evacuate.- MU Extension publication EMW1011, Family disaster plan
- Be informed.
Learn about possible hazards and how to respond to each of them. Find out where shelters operate in your community. Be aware of the local emergency messaging and alert systems. Learn about the emergency plans that have been established by your state and local government.
Additional information
- Radiation Protection (U.S. Nuclear Regulatory Commission)
- Radiation Emergencies (U.S. Centers for Disease Control and Prevention)
- Radiation Protection (U.S. Environmental Protection Agency)
- Extension Disaster Education Network
- Ready (Federal Emergency Management Agency)