Assessing the impacts of Fusarium head blight (FHB) or Scab, caused by Fusarium graminearum, begins with scouting for FHB disease symptoms during the growing season. Understanding damage levels prior to harvest can provide a critical first step to reducing yield losses from both damaged grain and Fusarium mycotoxin (deoxynivalenol [DON] or vomitoxin) contamination. In addition, regular scouting can provide valuable information regarding fungicide efficacy, especially if the same fungicide regimen is repeated year after year. Scouting for FHB can generally begin 2–3 weeks post flowering or 2–3 weeks post application of a fungicide intended for FHB suppression. To assess FHB levels in a field, complete the following steps:
Step 1

For every 10–20 acre section of field, walk in a “V” or “W” pattern and cut and collect a handful of about 10 wheat heads each from 10 points into a single bag for assessment (Figure 1). The best way to do this is to not visually select the heads as our eye is naturally drawn to those that may appear different. In this case, it may be those that are affected by the FHB pathogen. If we “blindly” collect the heads, we are less likely to bias the samples we select.
Be sure to appropriately label all bags according to field site or field section for reference after completing head collections. Sample bags can be easily transported to any location for further processing of steps 2 through 4. Ideally, samples should be kept refrigerated and processed within 24 hours of collection.
Step 2

From each collection bag, remove 100 wheat heads and note how many have visible symptoms of FHB (Figure 2). This would include the initial bleaching symptoms that can occur on single florets, spikelets, or even to whole heads. It does not matter how much of the head is affected, only if it is affected. This is known as the disease incidence, or percentage of heads infected in that section of the field, and will be a number from 0 to 100.
Step 3
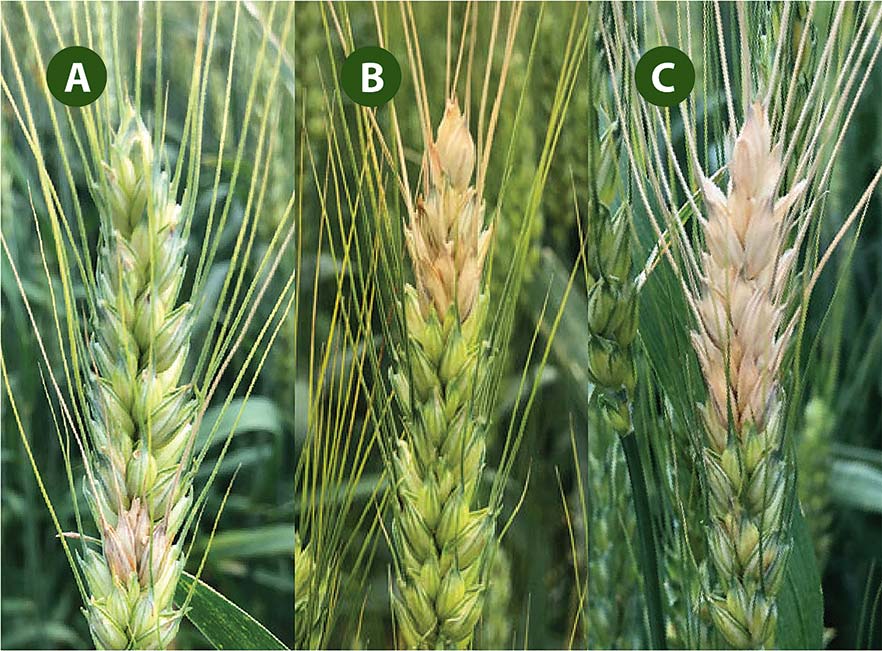
Next, set aside the heads that do not show any symptoms of FHB. Of the infected heads, how far has the infection spread within the head? On average, how much of the head is infected (Figure 3)? A single spikelet (A)? A quarter of the head (B)? Half of the head (C)? This is the percent disease severity and will be a number from 1 to 100.
Step 4
The final step is to calculate the FHB index value, a value that numerically represents the level of FHB in a particular section of a field. Simply, multiply disease incidence (Step 2) by disease severity (Step 3), then divide by 100 and you are left with what is known as FHB index. For example, if 25% of the heads are showing symptoms of infection and an average of 10% of the head is infected, the index value would be calculated as: FHB index = (25 × 10) / 100 = 2.5. A field with an FHB index value of 10 or greater is considered high. The higher the index value, the higher the disease and the higher the risk for losses attributed to FHB. There also is a relationship between FHB index and end of season deoxynivalenol (DON or vomitoxin) concentration in the grain. For this reason, it is important to understand which fields or field sections may have higher FHB index values for harvest considerations.
Harvest considerations
Kernels infected by FHB pathogens such as Fusarium graminearum are often lightweight and stick to chaff material. Turning up the blower and slowing down the combine speed can help to reduce the presence of some of these lightweight, contaminated kernels that might otherwise find their way into a grain load. In general, fields that have high levels of FHB should be harvested separately to reduce the possibility of introducing contaminated kernels into an otherwise sound load of grain.
Do not save grain with high levels of FHB. The pathogen can continue to grow on contaminated grain and DON levels can increase in the bin.
Understanding DON and when to test for it
One of the most important factors to consider when making FHB management decisions is DON contamination of the grain. Although DON can be present in the plant as early as when infection begins, DON accumulation in the grain and the associated tissues changes during the season. That is to say, a test for DON conducted at the Milk stage or Soft Dough stage of growth would not accurately reflect the final DON concentration in the grain at maturity. Testing for DON is most accurate when completed at harvest or grain maturity. Although there is no test that can predict DON concentration at maturity in season, calculating an FHB index value can serve as a valuable indicator for overall risk of Fusarium mycotoxin contamination.
Straw is also known to accumulate DON. Caution should be used in situations where straw is baled from heavily contaminated fields and is intended for use as bedding. This will increase risk of livestock exposure to Fusarium mycotoxins, especially when Fusarium-contaminated grain is used for feed in conjunction with contaminated straw.
By scouting for FHB and assessing FHB incidence, severity, and overall incidence on a field by field basis, adjustments can be made to harvest equipment to reduce yield loss due to grain damage and mycotoxin contamination.